
Living Well
The Hard Truth: Can You Really “Heal” Your Body Through Alkaline Diets?
Jul 25, 2016In today’s fast-paced world, we often forsake our health for convenience and speed. But in the long run these quick, short-term fixes may actually slow us down by contributing to disease, insulin resistance, bone loss, weight gain, low energy and pain.
One of the contributors to this may be low grade acidosis in the body. This is often expressed by people as a “pH level” being too acidic.
What Is ph balance diet ?
Acidity and alkalinity are expressed on the pH (potential of hydrogen) scale, which ranges from 0 (strongly acidic) to 14 (strongly basic or alkaline). A pH of 7.0, in the middle of this scale, is neutral.
Blood is normally slightly alkaline, with a normal pH range of 7.35 to 7.45. The body usually keeps the pH of blood in a very tight band, close to 7.40
“Body too Acidic”: Is It Real Or Hokum?
The idea of being ‘too acidic’ and how this could make you susceptible to disease has been around for a long time and is often discounted by medical doctors. They say that since the pH level of the blood (acidity or alkalinity measured through a blood test) shows no change, irrespective of the diet you eat, the idea of being ‘acidic’ must be false.
Where Else Could The Acidity Hit Us?
Doctors of naturopathy and alternative medicine swear by treating “acidosis” with the right diet, lifestyle and supplements. They say that while the body has extremely efficient buffering systems to maintain a stable blood pH, diet and lifestyle choices can affect the pH inside the cells, in the intracellular space. Low-grade acidosis in these areas can cause disruption of enzyme function, loss of insulin sensitivity, and cause cellular metabolic changes that lead to illness.
Are Some Foods Acidic And Some Alkaline?
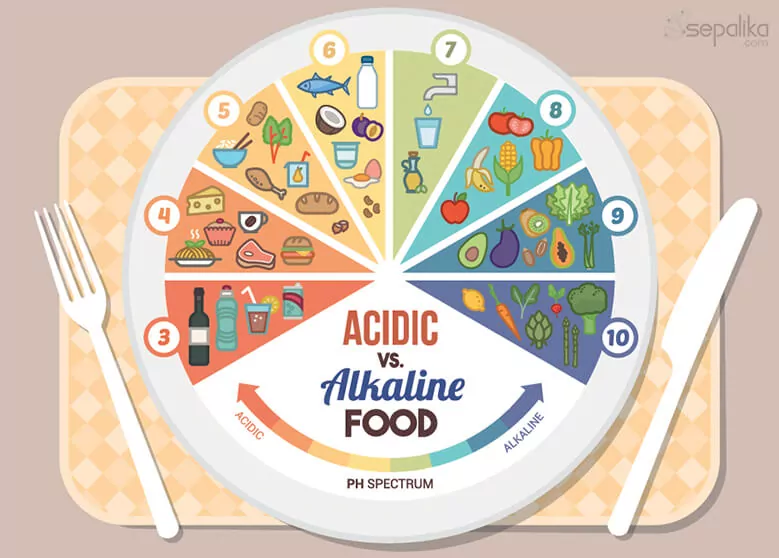
acidic v/s alkaline food
Yes, they are, to the extent that you can measure how acidic or alkaline a food is by say, taking a portion of it and analyzing it. Lemon juice is highly acidic, with a ph balance diet of 2.2, while Brussel sprouts can have a pH of around 6.4. However, when speaking about diet-induced low-grade acidosis, the discussion is no longer about the pH of the food or beverages consumed. It is about how these foods affect the acid/base levels in the intracellular spaces of the body.
What affects Acid/Alkaline Balance?
Diet and lifestyle factors play a major part in whether we function optimally and in balance or below par, with an overly acidic system.
In a contribution to the International Acid-Base Symposium, researcher Jürgen Bormann noted that, “although homeostatic mechanisms and the kidneys’ capacity to excrete acids can prevent strong diet-induced alterations in blood ph balance diet, even moderate increases in blood hydrogen ion levels (increased acidity) as a result of unfavorable diet composition can have long-term consequences for the occurrence and progression of a number of diseases”.
How Much Does The pH Need to Swing, to Have A Negative Impact On Health?
Studies show that the standard western diet tends to generate high levels of metabolic acids and inflammation; in part due to high salt intake, high processed foods and sugars and a reduced intake of dietary elements such as potassium, found in plant foods.
As mentioned earlier, except under extreme circumstances, even with our highly refined diet, blood pH is maintained within a “normal” range due to the body’s efficient balancing systems. However, though blood ph balance diet is maintained at a “safe” level, the body equilibrates closer to the more acidic level of the range instead of calibrating toward the more alkaline end of the normal range: this constant low-grade acidosis can have severe metabolic and health consequences if it remains for a long term.
Research suggests that diets which constantly generate a higher acid load, in conjunction with a natural age-related decline in kidney function, are the two main factors that render the body less capable of excreting metabolic acids, leading to a greater risk for the development of chronic metabolic disorders over time.
Aren’t There Some Areas Of My Body That Need to Be More Acidic?
Absolutely. The ideal pH varies throughout your body for many reasons. While we require body fluids such as blood and the fluids inside and between our cells to be more alkaline, areas such as the bowel and skin will be slightly more acidic which, amongst other things, helps keep unfriendly bacteria away. Then of course there is the hydrochloric acid in our stomach that needs to be highly acidic, with a ph balance diet of around 2.5, so that it is able to easily break down components in our foods.
Again, it’s a delicate balance; but overall the body requires a slightly alkaline environment, especially in it’s body fluids, in order to function optimally.
How Do Diet And Lifestyle Influence Acid-Base Equilibrium?
While optimal ph balance diet is well monitored and vital to maintain, we don’t always make it easy for our body to hold onto this balance.
The Standard American Diet (SAD) and lifestyle may be putting you at risk for chronic disease and an overly acidic system, due to it being:
- Filled with acid-forming processed foods; heavily processed grains, meats, dairy and sugars, and low in alkalizing vegetables.
- High in sugary drinks and low in water.
- High in salt.
- High in stress and pollution and low in clean air, exercise, sleep and calm.
How Does Low-Grade Acidosis Impact Health?
Decreased bone and muscle mass in adults is a well-documented consequence of severe metabolic acidosis. In the case of ongoing low-grade acidosis, the skeletal system continues its natural buffering process in order to maintain acid-balance homeostasis, a process that over time may become detrimental to bone mineral content and bone mass.
It has been demonstrated that even the slightest degree of metabolic acidosis produces insulin resistance in healthy humans and many recent epidemiological studies link metabolic acidosis indicators with both insulin resistance and systemic hypertension.
The strongly acidogenic diet consumed in developed countries produces a lifetime (slightly) acidotic state, exacerbated by excess body weight and aging, which may result in metabolic syndrome, and type 2 diabetes, contributing to cardiovascular risk.
Recent research has now shown that dietary acid load is the best predictor of kidney stones.
In 2008, a study examining the associations disorders in women, found that acidic dietary loads were positively associated with higher systolic and diastolic blood pressure, as well as increased LDL-cholesterol, basal metabolic index (BMI) and waist circumference.
How Do I know If My body Is too Acidic?
Symptoms often associated with low-grade acidosis include:
- Frequent Sighing
- Shortness of Breath
- Insomnia
- Water Retention
- Arthritis
- Headaches
- Kidney Stones
- Strong Perspiration
- Dry Hard Stools
- Foul-Smelling Stools and
- Halitosis.
Acid/alkaline tests can be bought cheaply from most drugstores, however using first morning urine is an inaccurate test to assess body and blood acid load.
THE FINAL VERDICT: Low grade acidosis is a real problem. Fortunately, with just a little bit of will and discipline, tackling it is fairly easy. Eating alkalizing foods and supplementing with the right vitamin and minerals can help you get to a ph balance diet and heal your body.
References:
- Adrogué HJ, Madias NE. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med 1981; 71:456.
- Balch P; Prescription for Nutritional Healing; 2010, Penguin, UK.
- Ball D, Maughan RJ; Human and Clinical Nutrition – Blood and urine acid–base status of premenopausal omnivorous and vegetarian women; British Journal of Nutrition / Volume 78 / Issue 05 / November 1997, pp 683-693; http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=887444&fileId=s0007114597001748
- Braun L, Cohen M; Herbs and Natural Supplements – An Evidence Based Guide; 2007; Elsevier.
- Frassetto LA, Morris RC, Jr, Sebastian A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am J Physiol Renal Physiol. 2007;293(2):F521–F525.
- Frings-Meuthen P1, Baecker N, Heer M; Low-grade metabolic acidosis may be the cause of sodium chloride-induced exaggerated bone resorption; J Bone Miner Res. 2008 Apr;23(4):517-24.
- http://www.ncbi.nlm.nih.gov/pubmed/18052757
- Jehle S, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006;17(1):3213–3222.
- Pan M, Meng Q, et al; Stimulation of intestinal glutamine absorption in chronic metabolic acidosis. Surgery 2004; 136:127-134
- Pizzorno J; Acidosis: An Old Idea Validated by New Research; Integr Med (Encinitas). 2015 Feb; 14(1): 8–12; http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4566456/
- Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–797.
- Schwalfenberg GK; The Alkaline Diet: Is There Evidence That an Alkaline pH Diet Benefits Health? J Environ Public Health. 2012; 2012: 727630. Published online 2011 Oct 12. doi: 1155/2012/727630 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3195546/
- Souto G, Donapetry C, Calviño J, Adeva MM. Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord. 2011;9(4):247–253. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3155690/
- Trinchieri A, Esposito N, Castelnuovo C. Dissolution of radiolucent renal stones by oral alkalinization with potassium citrate/potassium bicarbonate. Arch Ital Urol Androl. 2009;81(3):188–191.
- Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69(4):727–736.
- Vormann J, Remer T; 2008; Dietary, Metabolic, Physiologic, and Disease-Related Aspects of Acid-Base Balance: Foreword to the Contributions of the Second International Acid-Base Symposium; Supplement-Journal of Nutrition; http://jn.nutrition.org/content/138/2/413S.long
- Walker AF et al; Magnesium citrate found more bioavailable than other magnesium preperations is a randomized, double-blind study. Magnes Res 16.3 (2003): 183-91
- Welbourne T, Weber M, Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease; J Clinical Investigation; 1972; 51:1852-1860
- Wiederkehr M1, Krapf R. Metabolic and endocrine effects of metabolic acidosis in humans; Swiss Med Wkly. 2001 Mar 10;131(9-10):127-32. http://www.smw.ch/docs/pdf200x/2001/09/smw-09666.pdf





